Research and development center
Biological sample analysis and detection center
Biological sample analysis and detection center
Based on the GLP management system, the biological sample testing center is a third-party public service platform for providing standardized biological sample testing services. Center based on perfect quality management system and the GLP research for many years, equipped with complete facilities and international advanced equipment, USES the industry recognized biological analysis in laboratory information management system of Watson LIMS ™ to manage laboratory, its internal management, experiment method to form and content completely accords with the specification of GLP and 21 CFR PART11, provides a safe and reliable data for laboratory management; The center is dedicated to the analysis and testing of biological samples. It can develop, verify and apply a variety of analytical methods for testing, and provide professional technical services from a scientific and regulatory perspective.


We have a technical team with a PhD and master degree as the core, and we have more than 20 years of experience in biological sample analysis and the backbone of 3-4 years. The core members have rich experience in mass spectrometry test project and method development. Center staff have strong professional knowledge background and received systematic GLP training, is a professional team with strong quality.

Sun Jingjing, Ph. D.
• Doctor, with related research experience of roche genentech. Host and participate in national projects such as major new drug innovation projects. He has a number of new drug preclinical and clinical pharmacokinetic project research experience.

Yin Wei, Ph. D.
• Doctor, mainly carries out researches on proteomics and metabolomics based on lc-ms, focuses on biomarkers for early diagnosis of diseases, and has rich experience in the development and research of mass spectrometry methods.

Zhou Lanhua, M. S.
• Master degree, once engaged in biological sample analysis in pharmatech, independently undertook the domestic/international customer biological sample analysis project; Participated in dozens of bioanalytical/methodological validation projects; Participated in the on-site inspection of CFDA for many times.

Hu Zhongfang, M. S.
• Master, participated in the quality research of many new drugs, pharmacokinetics research of healthy human body, as well as the writing of application materials and the analysis and determination research of many drug applications.

Wu Yi, M. S.
• Master, main research direction is clinical data statistical analysis, familiar with medical, pharmaceutical and biological statistics and other relevant research methods, good professional skills in data processing and computer system management.

Xie Rui, M. S.
• Master, participated in LC/ ms-related drug metabolism, metabolomics and other related work in key laboratory of drug quality and safety early warning, ministry of education, China pharmaceutical university.
In the past three years, the company has undertaken nearly 20 evaluation projects, including the research on preclinical pharmacokinetics of a class of new drugs and generic drugs, preclinical pharmacokinetics research, bioequivalence evaluation project of generic drugs, etc. It has more than ten independently developed liquid and quality methods, and has applied for two patents and two government projects.
1、Qualification level recognized by authority
In 2017, the center successfully passed the capability verification program organized by the China food and drug testing institute. In June 2018, it passed the national GLP toxicokinetics certification inspection. In 2018, it passed the national inter-laboratory quality evaluation of clinical testing with full marks. The center has become the only biological sample testing institution in south China with GLP qualification and quality evaluation by the ministry of health.

2、Sound organizational structure and quality management system
According to NMPA's latest verification requirements and FDA/EMA/NMPA's biological sample analysis related guidelines and regulatory guidelines, guangzhou medical research institute biological sample testing center has established an independent and perfect functional department and organizational structure and formed a standardized laboratory quality management system. Nearly 120 existing system documents, the content covers comprehensive management, quality assurance, file management, biological analysis, instrument management five aspects, form a complete specification of the project management system, quality assurance system, instrument control system, the original data management system, personnel training system, biological sample management system, experiment material management and safety/emergency systems.
3、International advanced instruments and equipment
The laboratory management system WatsonTM LIMS is adopted by the center. Its built-in management form, experimental method and content fully comply with GLP and 21CFR PART11 specifications, can provide customers with safe and reliable data management, issued reports can support customers to make global declaration. The experimental equipment includes liquid-mass coupling analyzer, liquid workstation, high-speed refrigerating centrifuge, ultra-low temperature biological sample storage box and so on. The relevant equipment for biological sample testing has national certification qualification, and the operation of each instrument has a perfect SOP system and use and maintenance records.



4、Rich project experience
Over the past three years has been for nearly 20 evaluation projects, including a class of new drugs and generic drugs preclinical drug dynamics research, preclinical pharmacokinetic study, generics bioequivalence evaluation project, etc., to form mature project development process, have good capacity for independent development liquid qualitative analysis method and efficient project operation skills.
5、Complete professional service system
A complete set of professional service system can provide one-stop biological sample analysis services. Strict management of sample receiving and storage, experiment process strictly controlled by GLP standard, perfect QA review process, professional and industry-recognized data analysis and statistics system, traceable data record and backup system, summary report in line with international standards and regulations.
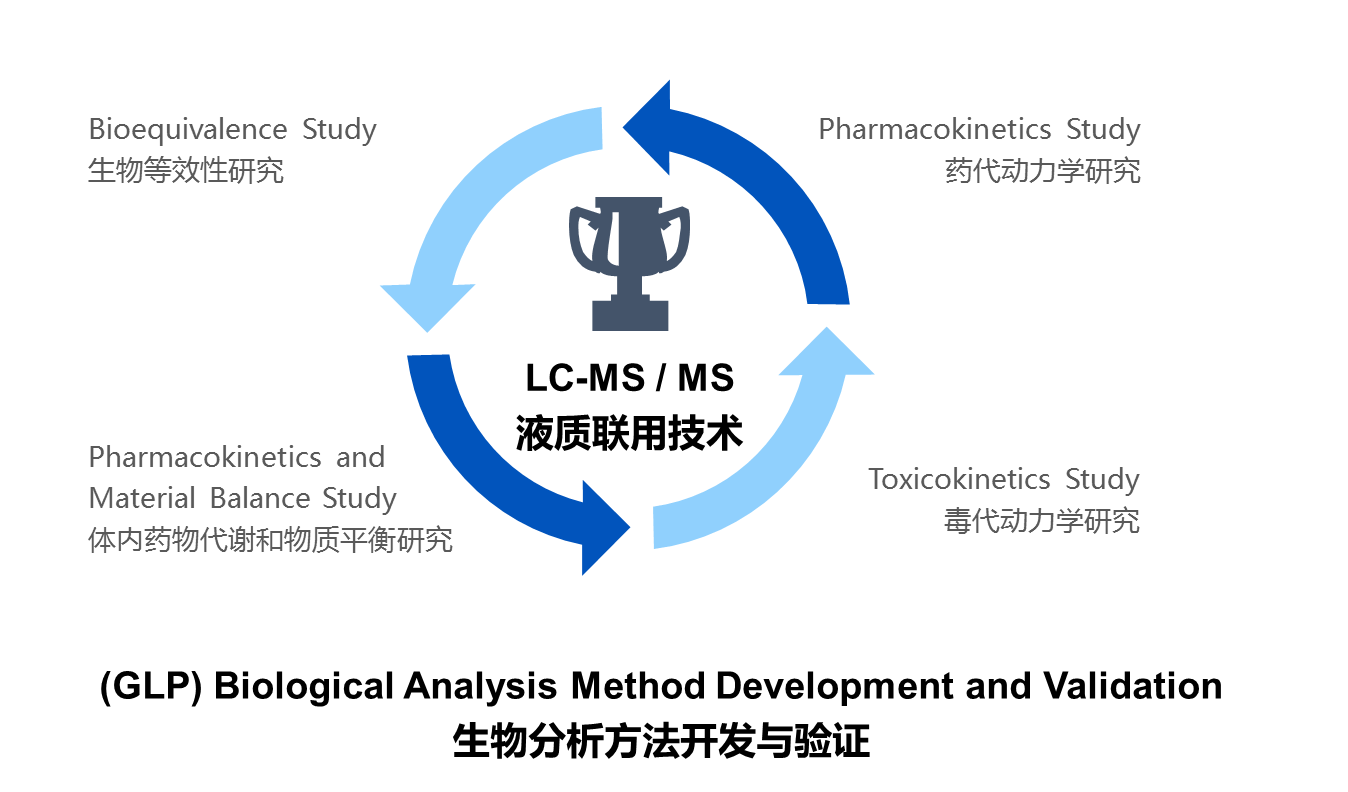
1. LC-MS /MS method development and methodology confirmation
2. Bioequivalence of generic drugs
3. Clinical biological sample analysis and detection of new drugs
4. Preclinical GLP test and early DMPK screening
5. Preclinical pharmacokinetic study
6. Outsourcing service (CRO)